it Was last week that the FDA (Food and Drug Administration), the body that controls the food and medicines in the USA, approved the MiniMed 670G, a device that allows you to automatically monitor glucose and it also provides the doses of insulin required.
This "new" innovative technology, that almost does not need the intervention of the patient to control the disease, you will reach the american market in general already in 2017.
Jeffrey Shuren, director of the Center for Devices and Radiological Health of the FDA, the MiniMed 670G will allow people with type 1 diabetes have a better life because the system will help, almost autonomous, to monitor the glucose levels and also to administer insulin.
The FDA is dedicated to making technologies available that can help improve the quality of life for those with chronic diseases – especially those that require day-to-day maintenance and ongoing attention
This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin
To Dr. Francisco Carrilho, the President of the board of the Portuguese Society of Endocrinology, Diabetes and Metabolism, this equipment is a hybrid system but that still is not fully automatic, this is because during the day the patient will need to enter some parameters related with the power for the device to calculate the amount of insulin to administer.
Francisco Carrilho, reveals that this is a disease that requires a lot of input from the patient, both to make the treatment as in the evaluation of treatment. On average, it takes four insulin injections per day.
according To the DN, in Portugal it is estimated that there are one million diabetics, of which 50 thousand have type 1 diabetes. Type 1 diabetes is a disease caused by the body that destroys the cells that produce insulin.
How to use the MiniMed 670G?
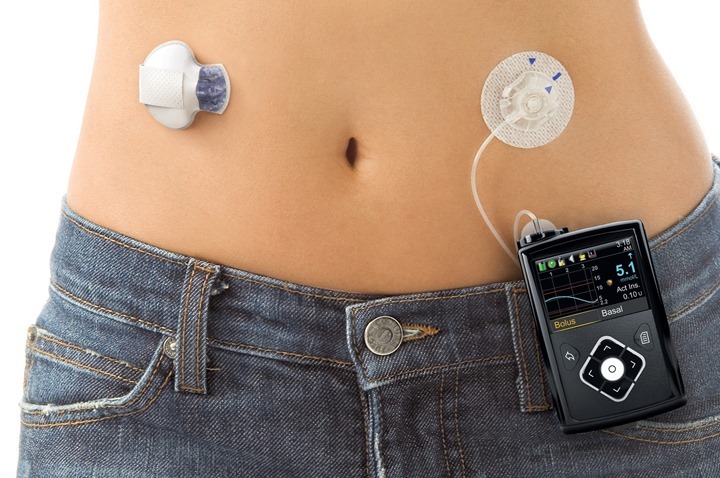
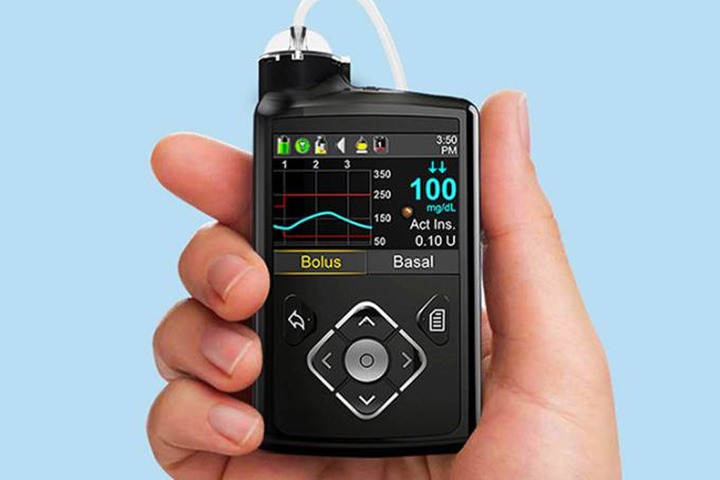
The MiniMed 670G, can be set, for example, on a belt and is connected to the body via a catheter to apply insulin. The evaluation of the rate of sugar in the blood of patients is carried out every five minutes.

Source:DN
This equipment has been the target of several tests and the results show that there may be some side effects such as hypoglycemia, hyperglycemia and even irritation of the skin. The MiniMed 670G is recommended to be used by people with over 14 years of age.
No comments:
Post a Comment